


Science with Sydney
Surface Tension
Surface tension of water is a result of something called "inter-molecular attraction". To understand what that is let’s first look at what water is made of. Some of the most basic building blocks of everything around us are molecules. Now, molecules are very, VERY, VERY small structures. These molecules are literally found in everything; you, me, that banana you ate for breakfast, the moon, the stars, and even the air we breathe. They are everywhere! Below is a diagram of what a water molecule is made of. If you look closely you can see that it has two “H’s” and one “O”. The (H) stands for hydrogen and the (O) stands for oxygen. In chemistry you would write this as H2O, you may have even heard someone call water H2O before, and that is because of the way chemists write the water molecule.

Now as we look closely at this water (H20) molecule you can see that there are (+) and (-) signs next to the H’s and O. These stand for positive (+) and negative (-) forces. You don’t need to worry too much about what those are now but just know that the positives (+) are attracted to the negatives (-). You can think of it like the (+’s) will only hold hands with the (–‘s)… and they always want to hold hands so they will do so whenever they can. This is what we are talking about when we talk about “inter-molecular attraction”, the molecules are attracted to one-another. Below you can see how all the positive (+) H's are connected to the negative (-) O's in the three water molecules and how they are holding hands.
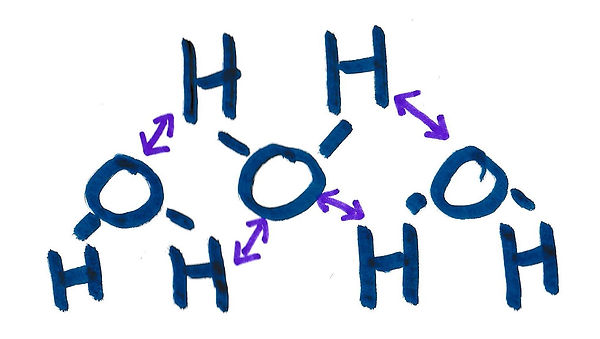

So now we understand that the water molecules (H20) are attracted to one-another and want to “hold hands” because of inter-molecular attraction. Now let’s watch the video below of Sydney doing an experiment that demonstrated surface tension and we will talk a little more after the video about why you are seeing what is happening.
So why did the water bubble up over the rim of the glass? You guessed it, because the water molecules (H20) were “holding hands” because of inter-molecular attraction on the surface of the water. They held on as tight as they could until the weight of the water on the inside pushing out was too much and broke the bond (pulled the hands apart) and the water trickled down the side of the glass.

Now it's your turn!
For this experiment you will need:
-
A jar of coins
-
A cup
-
Water
Do like Sydney and try to see how high above the rim of the cup you can get your water before it pours over the side.
Tip: try adding things to the water like soap to see if it makes a difference